


Submit
Accurately fill out case analysis form.

Analysis
Your case is reviewed in depth by an attorney within 2 business day.

Follow-Up
We will reach out directly to you discuss next steps.

Medtronic Infuse Lawsuits
The attorneys of the Infinity Law Center are currently evaluating potential lawsuits for patients who have experienced negative side effects from an Infuse Bone Graft.
Hundreds of lawsuits have been filed against Medtronic, the maker of Infuse Bone Graft, after patients suffered severe health complications. Patients who have suffered health complications should seek legal advice to determine whether or not financial compensation can be obtained.
Off-Label Use of Infuse Bone Graft Linked to Health Complications
Several hundred complaints have been submitted to the FDA involving health complications as a result of off-label use of Infuse Bone Graft. The FDA has only approved Infuse Bone Graft for a specific type of spinal surgery, tibia repairs, and dental procedures. Reports indicate that up to 80% of surgical procedures involving Infuse Bone Graft were off-label procedures.
When used in off-label procedures, Infuse Bone Graft has been linked to serious side effects. According to the FDA, the most common side effects include swelling in the neck, difficulty breathing, and difficulty swallowing. However, the following severe side effects have also been reported:
- Cyst Formation
- Infertility in men
- Cancer
- Uncontrolled bone growth
FDA Warns Consumers About Infuse Bone Graft Complications
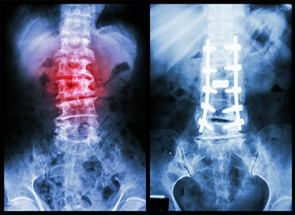
The growing number of complaints from patients injured by Infuse Bone Graft forced the FDA to release a warning to alert customers that off-label use of Infuse Bone Graft could cause serious complications, especially when used for off-label purposes.
The FDA announced that swelling in the neck and throat was the most common complication but there were several other reported complications that required patients to undergo emergency surgery.
Justice Department Investigation
After the FDA released a statement, the U.S. Justice Department launched an official investigation. The investigation found that up to 80% of surgical procedures using Infuse Bone Graft were for off-label purposes. In addition, the investigation released a previous settlement in which Medtronic paid $10 million dollars in a whistleblower suit that alleged that Medtronic gave inappropriate payments and referrals fees to doctors and surgeons for recommending Medtronic for off-label purposes.
Despite the evidence the Justice Department uncovered, no criminal or civil charges were filed and the case was closed. However, it still exposed Medtronic’s unethical business practices.
U.S. Senate Investigation Uncovers More Unethical Business Practices

After further complaints, the U.S. Senate Finance Committee launched their own investigation into Medtronic. Their investigation uncovered a $200 million dollar payout in consulting fees to Medtronic-paid study authors when the authors were supposed to be objectively examining the effectiveness and safety of Infuse Bone Graft.
Medtronic Infuse Bone Graft Lawsuits
Due to the lack of warning given to patients and the promotion of Infuse Bone Graft for off-label purposes by Medtronic, several lawsuits have been filed against the company. These lawsuits claim that:
- Medtronic did not warn consumers about the serious complications of Infuse Bone Graft.
- Medtronic gave inappropriate payments and referrals fees to doctors and surgeons to use Infuse Bone Graft for off-label purposes, despite knowing the risks.
- Advertisements claimed Infuse Bone Graft was safe, yet Medtronic knew there were potential complications.
Do You Have an Infuse Bone Graft Claim?

If you or a loved one has been injured as a result of an Infuse Bone Graft surgery, then take a moment to fill out our no-obligation case evaluation form. The experienced and dedicated attorneys of Infinity Law Center will review the details of your case and determine whether or not you have a claim.
You or a loved one did not deserve to be injured as a result of Infuse Bone Graft surgery. Our experienced attorneys of the Infinity Law Center will fight to get you the compensation you rightfully deserve for your injuries.
ATTORNEY ADVERTISEMENT DISCLAIMER
This website may be considered Attorney Advertising in some jurisdictions. In such jurisdictions, prior results do not guarantee a similar outcome. This website is not affiliated in any way with the following: Infuse Bone Graft is a registered trademark of Medtronic, Inc. Consult your doctor or physician before starting or stopping any medications. This site does not provide medical or legal advice, and services are not available in all states.
Copyright © 2014-2022 Infinity Law Center






